Case study for the European Commission – Joint Research Centre
Project:
 The Joint Research Centre (JRC) provides support to EU policies by providing independent scientific advice to the Commission, the European Parliament, the Council of Ministers and the Member States. One of the JRC activities related to chemicals is concerned with the development, validation and dissemination of quantitative structure-activity relationships (QSARs) with the overall aim of promoting the availability of scientifically valid QSARs for regulatory use.
The Joint Research Centre (JRC) provides support to EU policies by providing independent scientific advice to the Commission, the European Parliament, the Council of Ministers and the Member States. One of the JRC activities related to chemicals is concerned with the development, validation and dissemination of quantitative structure-activity relationships (QSARs) with the overall aim of promoting the availability of scientifically valid QSARs for regulatory use.
The need for scientifically valid QSARs has increased in recent years, in particular as a result of the new REACH (Registration, Evaluation and Authorisation of Chemicals) Regulation.
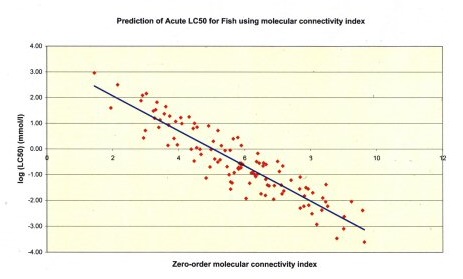
In 2005 a project titled “Validation of quantitative structure-activity relationships for toxicological endpoints of regulatory importance; QSARs for acute toxicity to fish” was instigated. The overall objective of this project was to identify a short-list of promising QSARs for acute toxicity to fish, and to provide sufficient information on each short-listed QSAR to enable its scientific validity to be established.
Contribution:
BRE was appointed to carry out the work. The project was carried out in two parts.
- Preliminary review of available QSARs.
- Identification and validation of the most promising QSARs.
The preliminary review identified 81 sources of QSARs (containing 578 individual QSARs equations). Criteria were developed for assessing the reliability of each of these QSARs and each source was ranked against these criteria.
In the second part of the study, seven sources of QSARs were selected for a more in-depth validation exercise. The sources chosen were representative of the entire range of the ranking. The objective of this part of the study was to a) confirm the validity of the QSARs that were already highly ranked and b) determine whether the validity of the lower ranked QSARs could be improved. This allowed the key factors that need to be considered when validating QSARs for acute fish toxicity to be identified.
Benefits:
Reliable methods for predicting toxicity to aquatic organisms are becoming of increasing importance in a regulatory context. For example, under the REACH Regulation it is anticipated the QSARs will be used extensively in the interest of minimising animal testing. QSARs are likely to play an important role in the assessment of chemicals produced or imported in relatively small quantities, for which minimal animal testing is foreseen, and in a supporting role for higher tonnage chemicals.
